Prologue
The mound beside the temple graced his seat. And the evening lights of the village from a vantage point set the scene for Zen Master’s daily contemplation.
Ma cautiously approached and sat beside him.
The dim evening lights of the village invited their thoughts and minds.
“How was your day?” asked Zen Master
Trying to recollect his memories,
Ma replied with a smile
“You train me well.”
And after a pause, he continued
“I will remember everything you teach me and one day I will become a Zen Master just like you”
“Can you remember everything?” asked Zen Master realizing the importance of his training
Ma tried to understand the tenor of the question in his mind.
“Can I?” inquired Ma with hesitation, quickly giving up his thoughts.
Immunological memory
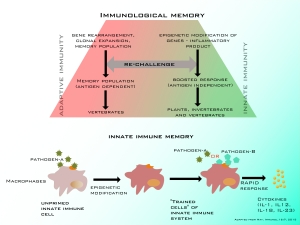 Memory in our immune system is indispensable. Immunological memory is the ability of the immune system to respond more rapidly and efficiently to pathogens that have been previously exposed. Threat from invading pathogens and environmental triggers (henceforth antigens) are constant to the host. And to cope with these threats, immunological memory is pivotal in its recognition and elimination.
Memory in our immune system is indispensable. Immunological memory is the ability of the immune system to respond more rapidly and efficiently to pathogens that have been previously exposed. Threat from invading pathogens and environmental triggers (henceforth antigens) are constant to the host. And to cope with these threats, immunological memory is pivotal in its recognition and elimination.
Based on the ability to respond to antigenic challenge and contingent on
- Specificity and
- Clonal selection (theory), which leads to Immunological memory, the immune system is classified into innate and adaptive immunity.
When encountered with an antigenic challenge, adaptive immune cells such as T or B lymphocytes adapt (hence the name adaptive) in response to the antigen. This response is specific and as one of the outcome, memory population (to that specific antigen) are generated. And when re-challenged, the memory response is rapid and robust.
However, innate immune cells such as granulocytes, macrophages, monocytes and Natural Killer (NK) cells were thought to be completely non-specific and lacked memory response.
Identification of pathogen pattern recognition (PPR) receptors in innate immune cells has reasoned the complete non-specificity dogma. It is now appreciated that innate immune system has a semi-specific recognition of pathogen associated molecular patterns (PAMPS) from different classes of microbial pathogens. And now, experimental studies and proposed concepts stack up in support of innate immune memory.
Innate immunologists argue that epigenetics is the key that unlocks the memory of innate immune system!
Epigenetics ”train(ed) innate immunity”:
Epigenetics focuses on cellular and physiological phenotypic changes that are caused by external or environmental factors. In essence, these factors turn on or off the gene expression and affect how the cells read genes instead of being caused by changes in DNA sequence.
When innate immune cells (such as macrophages) are treated with fungal structure β-glucan, positive epigenetic modifications occur in histone regulatory proteins (H3K4me1, H3Kme3 and H3K27ac). These changes are responsible for increased response with gene expression of host defense molecules in innate immune cells when re-stimulated with same or similar stimuli. Innate Immunologists argue that, epigenetic alterations due to exposure of pathogens in innate immune cells in vertebrates can equip them with an “antigen independent memory”. So when re-exposed to same or similar pathogens, there is heightened response. They propose such memory as “trained immunity”.
They insist that epigenetics in innate immune cells leading to memory response makes sense at an evolutionary stand point. Evolutionary conservation theory of “systemic acquired resistance” is proposed in innate immunity. This stems from the fact that innate immune memory is seen as host defense feature in organisms such as plants and invertebrates that lack adaptive immunity (Conventional adaptive immune memory is present only in vertebrates).
Furthermore, role of epigenetic modification in innate immune memory have also been shown in NK cells. Studies have shown that these ‘memory’ NK cells can rapidly degranulate and produce inflammatory cytokines upon reactivation.
It is proposed that these epigenetic modifications of cellular function is at the core of long term training of immunity for innate immune cells.
Other proposed mechanisms that are thought to play a role in innate immune memory include Long-term regulation of Non-Coding Ribo-Nucleic Acids (lncRNA) and metabolic pathways including glycolysis and Warburg metabolism are implicated.
Is memory forever?
Given the specificity and gene rearrangement with central and effector memory cells, adaptive immunity is for lifetime.
How long does the ”trained” innate immune cells have memory? Whether the altered epigenetics is retained after proliferation? Can it be transmitted epigenetically in the germ line (as seen in plants)? are important outstanding questions that remain to be answered.
In summary, classical adaptive immune memory is specific, antigen dependent and is mediated by gene rearrangements. Innate immune cells were thought to be completely non specific which lacked memory response. However, recent studies show that innate immune cells can be trained to acquire memory. Trained innate immune memory is semi-specific, antigen independent and can be mediated through epigenetic reprogramming.
Epilogue
Realizing the confusion in Ma’s voice,
Zen Master filled the silence with his consolations
“Train your mind! Be open to all the environment has to offer. Absorb the harmonious changes and try to preserve the experience in your memory. Your trained mind will adapt as need arises!”
“But, will the environment make me a Zen Master like you?” asked Ma
Waiting for an affirmation from his adoring master, his wanting eyes gazed at Zen Master
His glance spoke thousand words to Ma and he returned his gracious
Smile!
References:
- Murphy, K. M. (2012). Janeway’s Immunobiology (pp. 1–892). Garland Science.
- Netea, M. G., Latz, E., Mills, K. H. G., & O’Neill, L. A. J. (2015). Innate immune memory: a paradigm shift in understanding host defense. Nature Publishing Group, 16(7), 675–679. http://doi.org/10.1038/ni.3178

